Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kondo, Tomomi; Sasaki, Takehiko*; Ruiz-Barragan, S.*; Ribas-Ari o, J.*; Shiga, Motoyuki; Ruiz-Barragan, S.*
o, J.*; Shiga, Motoyuki; Ruiz-Barragan, S.*
Journal of Computational Chemistry, 42(3), p.156 - 165, 2021/01
Times Cited Count:4 Percentile:20.61(Chemistry, Multidisciplinary)We propose a canonical sampling method to refine metadynamics simulations a posteriori. This approach could be useful particularly when two or more free energy barriers are to be compared among chemical reactions in different or competing conditions. The method was then applied to study the acid dependence of polyalcohol dehydration reactions in high-temperature aqueous solutions. It was found that the reaction proceeds consistently via an S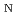 2 mechanism, whereby the free energy of protonation of the hydroxyl group created as an intermediate is affected significantly by the acidic species.
2 mechanism, whereby the free energy of protonation of the hydroxyl group created as an intermediate is affected significantly by the acidic species.
Chang, Y. L.*; Sasaki, Takehiko*; Ribas-Ari o, J.*; Machida, Masahiko; Shiga, Motoyuki
o, J.*; Machida, Masahiko; Shiga, Motoyuki
Journal of Physical Chemistry B, 123(7), p.1662 - 1671, 2019/02
Times Cited Count:4 Percentile:11.33(Chemistry, Physical)Dehydration of biomass-derived polyalcohols has recently drawn attention in green chemistry as a prototype of selective reactions controllable in hot water or hot carbonated water, without any use of organic solvents or metal catalysts. Here, we report a free-energy analysis based on first-principles metadynamics and blue-moon ensemble simulations to understand the mechanism of competing intramolecular dehydration reactions of 1,2,5-pentanetriol in hot acidic water. The simulations consistently predict that the most dominant mechanism is the proton-assisted S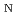 2 process, where the protonation of the hydroxyl group by water and the C-O bond breaking and formation occur in a single step. The detailed mechanism found from the simulations shows how the reaction paths are selective in hot water and why the reaction rates are accelerated in acidic environments, thus giving a clear explanation of experimental findings for a broad class of competing dehydration processes of polyalcohols.
2 process, where the protonation of the hydroxyl group by water and the C-O bond breaking and formation occur in a single step. The detailed mechanism found from the simulations shows how the reaction paths are selective in hot water and why the reaction rates are accelerated in acidic environments, thus giving a clear explanation of experimental findings for a broad class of competing dehydration processes of polyalcohols.
Ruiz-Barragan, S.*; Ribas Ari o, J.*; Shiga, Motoyuki
o, J.*; Shiga, Motoyuki
Physical Chemistry Chemical Physics, 18(47), p.32438 - 32447, 2016/12
Times Cited Count:8 Percentile:32(Chemistry, Physical)The use of high-temperature liquid water (HTW) as a reaction medium is a very promising technology in the field of green chemistry. In order to fully exploit this technology, it is crucial to unravel the reaction mechanisms of the processes carried out in HTW. In this work, the reaction mechanism of 2,5-hexanediol dehydration in HTW has been studied by means of three different ab initio simulations: string method, metadynamics and molecular dynamics in real time. It is found that the whole reaction involving the protonation, bond exchange and the deprotonation occurs in a single step without a stable intermediate. The hydrogen bonded network of surrounding water has a vital role in assisting an efficient proton relay at the beginning and at the end of the reaction. It is confirmed that the reaction is energetically most favorable in the SN2 pathway with an estimated barrier of 36 kcal/mol, which explains the high stereo selectivity and the reaction rate observed in experiment.